SCO-088, a novel and highly selective tyrosine kinase inhibitor (TKI) active in wild-type and mutant BCR ABL1, is being developed to treat resistant Chronic Myeloid Leukemia (CML).
Patients stop responding to available treatments or develop side effects. There are limited treatment options for CML patients who become resistant or intolerant to three or more TKIs. SCO-088 offers an investigational TKI treatment option in this setting.
SCO-088 has been investigated in healthy volunteers and CML patients in clinical trials. The Phase 1 study (CLR_15_03: Part A) in healthy volunteers established oral bio-availability of SCO-088 and once-a-day dosing. SCO-088 was well-tolerated in healthy volunteers, with no serious adverse events reported.
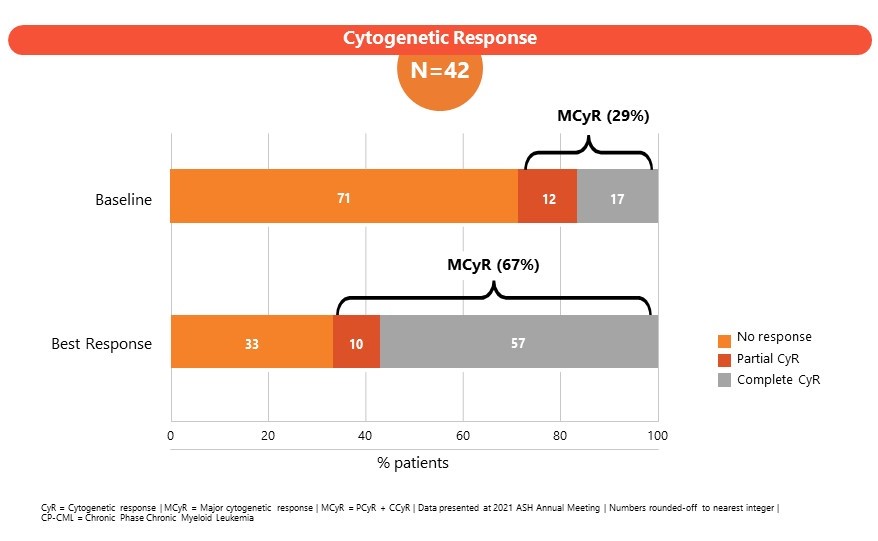
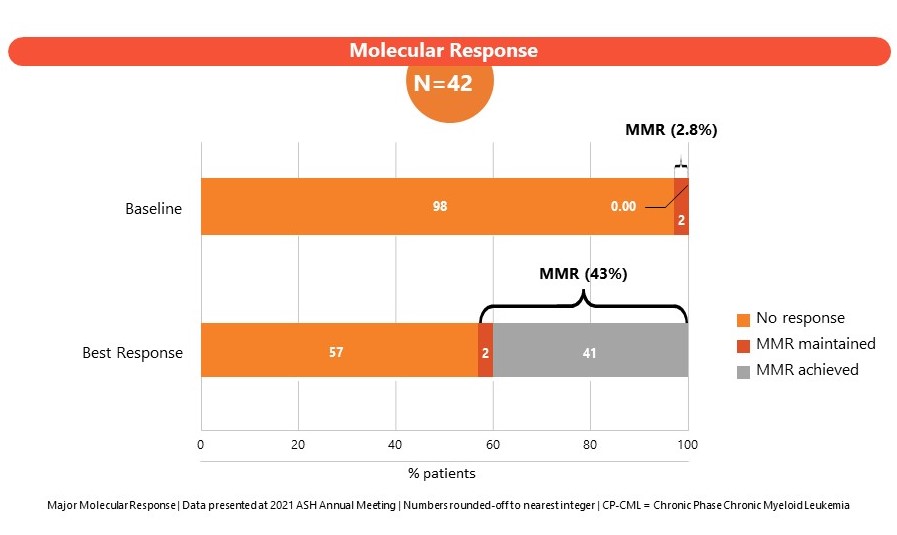
The Phase 1 study (CLR_15_03: Part B) in patients enrolled CML subjects failing 3 or more lines of TKI therapies or subjects ineligible for TKI therapy. A dose of 204 mg once daily was established as the maximum tolerated dose (MTD). SCO-088 has been well-tolerated with limited non-hematologic and cardiovascular side effects. Results of the Phase I study were presented at the American Society of Hematology, Annual Meeting 2022 and at the J Goldman CML symposium 2022.